


#ATOMIC PACKING FACTOR FOR BCC AND FCC PDF#
APPSC Lecturer (Polytechnic Colleges) Held on March 2020 Download PDF Attempt Online. Compute and compare its theoretical density with the.

Iron has a BCC crystal structure, an atomic radius of 0.124 nm, and an atomic. Show that the atomic packing factor for BCC is 0.68.
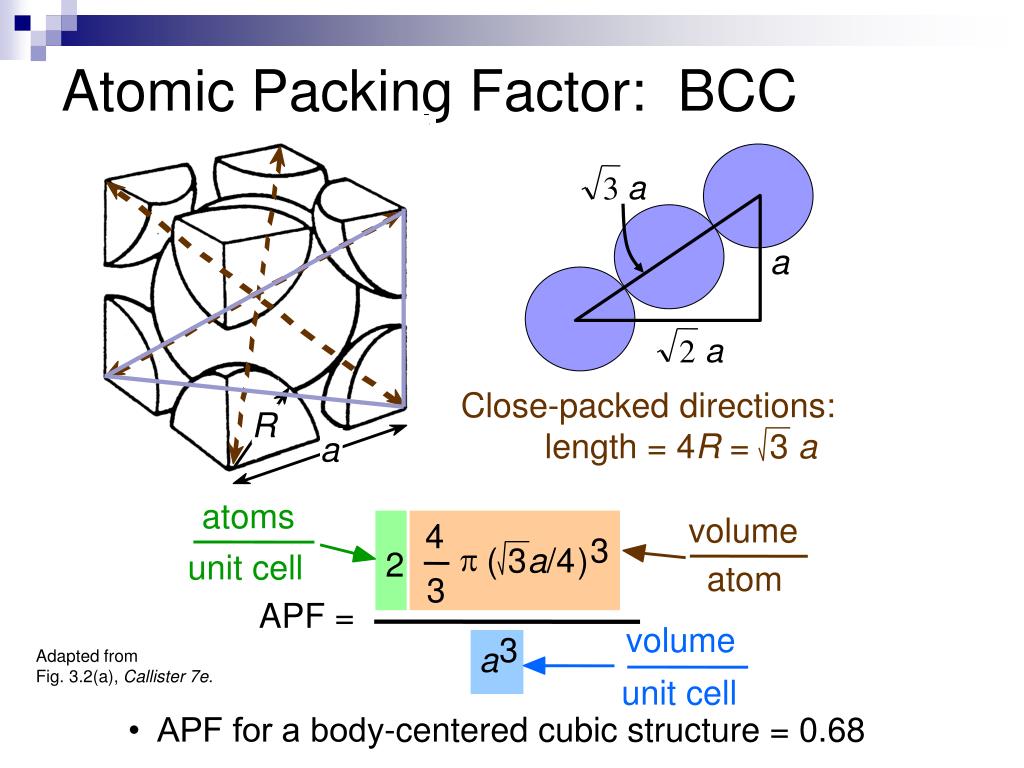
Calculate the theoretical density for Sr. Atomic packing factor for a FCC structure is: This question was previously asked in. Show that the atomic packing factor for FCC is 0.74. Strontium (Sr) has an FCC crystal structure, an atomic radius of 0.215 nm, and an atomic weight of 87.62 g/mol. Show that the atomic packing factor for BCC is 0.68 Show that the atomic packing factor for HCP is 0.74. For a simple cubic lattice, the packing efficiency is 52.4% and the packing efficiency is 68% for a body-centered cubic lattice or bcc. Chemical Engineering questions and answers. The packing efficiency of both of these is 74% which means 74% of the space is filled.
#ATOMIC PACKING FACTOR FOR BCC AND FCC HOW TO#
The cubic closed packed or ccp and the hexagonal closed packed or hcp are two efficient lattices when we consider packing. What is the Atomic Packing Factor How to calculate the Atomic Packing Factor for face-centered cubic (FCC) and body-centered cubic (BCC) crystal structure.A. It is dimensionless and always less than unity. This unit cell of the lattice is a three-dimensional structure that has one or more atoms and also void spaces irrespective of the packing present. Atomic Packing factor for SC BCC FCC and HCP In crystallography, atomic packing factor (APF), packing efficiency or packing fraction is the fraction of volume in a crystal structure that is occupied by constituent particles. When it is represented as a percentage then the percentage of the space applied by the constituent particles out of the total space present in the structure is called the packing efficiency of the unit cell.Ī lattice is largely made of a number of unit cells in which the lattice point is filled or occupied by a constituent particle. This can be obtained by dividing the volume of the constituent particles filled in the space by the total volume of the cell. The fraction of total space that is filled with the particular cell or structure is called the packing fraction. The space is filled by other constituents or particles. The fcc vaIue is the highst theoretically possible vaIue for any. Even when there is packing in the cell, a certain void is present in it. Similarly, in bcc lattice, th atomic packing factr is 0.680, and in fcc it is 0.740. Unit cell can be defined as a three-dimensional structure that is made of one or more than one atom. In hcp and ccp i.e., Face Centred Cubic StructureĪgain, we draw the face diagonal and as shown in the figure, In this case, since atoms are on the corners and an atom is present in the center, we draw a diagonal, and its length (c) can be calculated using Pythagoras theorem.Īnd since radius = 4 X Diagonal (as shown in the figure under Body Centred Cubic Lattice) 27 Related Question Answers Found Is hcp and bcc same The hexagonal closest packed (hcp) has a coordination number of 12 and contains 6 atoms per unit cell. When this is shown as a percentage i.e., out of the total space, the percentage that is held up by constituent particles is called the Packing Efficiency of a Unit Cell. Face-centered cubic (FCC): 0.74 (also called cubic close-packed, CCP) Body-centered cubic (BCC): 0.68. It can be obtained by dividing the total volume occupied by constituent particles by the cell's total volume. APF for the simple cubic structure 0.52 ATOMIC PACKING FACTOR (APF) contains 8 x 1/8 1atom/unit cell Lattice constant close-packed directions a R0.5a. The fraction of total space that is filled with the inherent constituent particles of a particular cell or structure is called the packing fraction. Some void space is always present irrespective of the type of packing the cell has.


 0 kommentar(er)
0 kommentar(er)
